

About Us
OVERVIEW
The Brain Stimulation and Therapeutic Modulation (BSTM) Division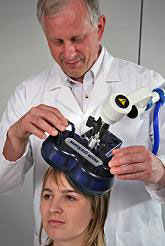 specializes in the use of emerging electromagnetic means of modulating brain function to study and treat psychiatric disorders. The BSTM encompasses research programs (preclinical, translational, and clinical), educational activities, and clinical services at NYSPI and NYPH utilizing existing and emerging brain stimulation and neuromodulation interventions in psychiatry. Available brain stimulation techniques include deep brain stimulation (DBS), modifications of electroconvulsive therapy (ECT), magnetic seizure therapy (MST), transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS). These techniques are applied alone as probes or therapeutic interventions, or in conjunction with functional imaging (simultaneous TMS/fMRI, TMS/PET, TMS/MRS). In addition to these stimulation and stimulation/imaging tools, the BSTM offers expertise in the neuroanatomical, cognitive and neurophysiological assessment of the impact of stimulation paradigms on brain function in pre-clinical and clinical settings. Our collaborations with the neuroanatomical expertise of Dr. Arango and neuropathological studies of Dr. Dwork provide the platform for studies on the impact of brain stimulation on hippocampal plasticity (including neurogenesis, synaptic remodeling, and gene expression). The collaboration with Dr. Herb Terrace, provides custom designed neurocognitive batteries to assess the impact of brain stimulation on a range of cognitive functions (including anterograde and retrograde amnesia, working memory, spatial memory, serial list learning, ordinal position, numerosity, and meta-cognition). The collaboration with Dr. Charles Schroeder provides expertise in intracerebral recordings to study the neurophsyiological effects of brain stimulation.
specializes in the use of emerging electromagnetic means of modulating brain function to study and treat psychiatric disorders. The BSTM encompasses research programs (preclinical, translational, and clinical), educational activities, and clinical services at NYSPI and NYPH utilizing existing and emerging brain stimulation and neuromodulation interventions in psychiatry. Available brain stimulation techniques include deep brain stimulation (DBS), modifications of electroconvulsive therapy (ECT), magnetic seizure therapy (MST), transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS). These techniques are applied alone as probes or therapeutic interventions, or in conjunction with functional imaging (simultaneous TMS/fMRI, TMS/PET, TMS/MRS). In addition to these stimulation and stimulation/imaging tools, the BSTM offers expertise in the neuroanatomical, cognitive and neurophysiological assessment of the impact of stimulation paradigms on brain function in pre-clinical and clinical settings. Our collaborations with the neuroanatomical expertise of Dr. Arango and neuropathological studies of Dr. Dwork provide the platform for studies on the impact of brain stimulation on hippocampal plasticity (including neurogenesis, synaptic remodeling, and gene expression). The collaboration with Dr. Herb Terrace, provides custom designed neurocognitive batteries to assess the impact of brain stimulation on a range of cognitive functions (including anterograde and retrograde amnesia, working memory, spatial memory, serial list learning, ordinal position, numerosity, and meta-cognition). The collaboration with Dr. Charles Schroeder provides expertise in intracerebral recordings to study the neurophsyiological effects of brain stimulation.
Research Activites & Grants
Active research in the BSTM spans basic neuroscience studies on brain behavior relationships, to novel intervention development, to clinical trials. A major focus of the work has been on the development of magnetic seizure therapy (MST) as a less invasive means of performing convulsive therapy. Specifically, MST can induce seizures from focal regions of the cortex that do not involve deeper brain structures (such as medical temporal cortex) that are implicated in the amnestic side effects of ECT. MST was conceived of and developed by our team, and parallel studies in a preclinical model (supported by NIMH R01 MH60884) and in patients with depression (supported by NARSAD, Stanley Foundation, and American Federation for Aging Research) have tested its feasibility and safety. Major results coming from the laboratory in the last 2 years include key publications on the safety of MST (and ECT) in a preclinical model (American Journal of Psychiatry), the finding that MST has a better acute safety profile than ECT in patients with depression (Neuropsychopharmacology), and neurophysiological evidence that MST-induced seizure are more focal and result in relative sparing of deeper brain structures compared to ECT (Clinical Neurophysiology). Our lab also found that ECT significantly induces the proliferation of new cells and aberrant sprouting of mossy fibers in the dentate gyrus in a preclinical model, while MST does not. These results have implications for the mechanisms of action of convulsive therapy, and for antidepressant pathways in general. We recently completed the first trial of the antidepressant efficacy of MST in patients with major depression, and presented these results as at the ACNP Hot Topic session. We are now engaged in a Stanley supported 2 center trial of MST in the US, and an international cooperative trial with sites in Wales and Scotland (supported by the Medical Research Council Brain Sciences II).
and in patients with depression (supported by NARSAD, Stanley Foundation, and American Federation for Aging Research) have tested its feasibility and safety. Major results coming from the laboratory in the last 2 years include key publications on the safety of MST (and ECT) in a preclinical model (American Journal of Psychiatry), the finding that MST has a better acute safety profile than ECT in patients with depression (Neuropsychopharmacology), and neurophysiological evidence that MST-induced seizure are more focal and result in relative sparing of deeper brain structures compared to ECT (Clinical Neurophysiology). Our lab also found that ECT significantly induces the proliferation of new cells and aberrant sprouting of mossy fibers in the dentate gyrus in a preclinical model, while MST does not. These results have implications for the mechanisms of action of convulsive therapy, and for antidepressant pathways in general. We recently completed the first trial of the antidepressant efficacy of MST in patients with major depression, and presented these results as at the ACNP Hot Topic session. We are now engaged in a Stanley supported 2 center trial of MST in the US, and an international cooperative trial with sites in Wales and Scotland (supported by the Medical Research Council Brain Sciences II).
Our ongoing work with subconvulsive levels of TMS encompasses basic studies using TMS in conjunction with functional imaging as a mapping tool, and clinical trials in the treatment of depression and other disorders. One of our basic cognitive neuroscience projects funded by DARPA utilizes fMRI and TMS in the study of the effects of sleep deprivation on working memory circuits. This work has isolated brain networks that are expressed during task performance, affected by sleep deprivation, and differentially affected as a function to cognitive susceptibility to sleep deprivation. Our 2 other DARPA grants examine field implementation strategies for TMS and the use of TMS in the study of deception. We recently completed the industry-sponsored (Neuronetics) pivotal multicenter trial of TMS in the treatment of depression, the results of which will be announced later this month. We are midway through a similar NIMH sponsored multicenter trial of TMS for depression (NIMH R01 MH069895). Our NARSAD funded clinical trial of TMS in the treatment of schizophrenia generated the pilot data for Dr. Stanford’s K23 application that received a score of 184 on first submission and supported Dr. Stanford’s selection as Janssen Translational Neuroscience Fellow. We also have active clinical trials on TMS in the treatment of OCD, tDCS for depression, and 3 contracts from Cyberonics. Two of these are multicenter postmarketing trials [Treatment Resistant Depression Registry and D21 Dose Finding Study], and one is an investigator-initiated study of the impact of VNS on TMS measures of cortical excitability.
In the last round we submitted an R21on the design of a novel TMS device with more physiologically informed pulse characteristics with collaborators from Magstim Company and the inventors of TMS at the University of Sheffield (Dr. Anthony Barker), a LRP and NSF Dissertation Support Award. For the next round we are preparing an SBIR with Neuronetics Company on the design of a novel MST device, an R01 with Sergievsky Center on fMRI/TMS studies of working memory, 3 new K Award applications, an R21 on maintenance TMS  following effective treatment with ECT, and an R03 on fMRI/TMS studies of language processing. We have also joined the NIMH Collaborative Project formerly named CORE (Consortium for Research on ECT) and are preparing with this group a competing renewal with a revised focus, now called PRIDE (Preventing relapse in depressive episodes).
following effective treatment with ECT, and an R03 on fMRI/TMS studies of language processing. We have also joined the NIMH Collaborative Project formerly named CORE (Consortium for Research on ECT) and are preparing with this group a competing renewal with a revised focus, now called PRIDE (Preventing relapse in depressive episodes).
In addition to its own internally generated research projects, the BSTM serves as a resource and collaborator for other groups wishing to utilize brain stimulation techniques to investigate other research questions. We have active collaborations with the Sergievsky Center in Neurology (DARPA funded), Barnard Psychology (DARPA), fMRI Center with Joy Hirsch, Hatch Center with Truman Brown (DARPA), Columbia Psychology (Tor Wager and Ed Smith), Larry Kegeles and Dikoma Shungu (Dana funded TMS/MRS study), the Anxiety Disorders Group (Liebowitz, Fallon, Simpson), and intramural NINDS (DARPA).
Facilities:
The clinical research facilities include the Brain Behavior Clinic, 2 Human TMS treatment suites, the TMS Unit of the fMRI Center (NI), and Stimulation/Imaging (TMS/fMRI, TMS/PET, TMS/MRS). The preclinical research facilities include pre-clinical stimulation studies, procedure suites, and the electronics shop specializing in novel device design and implementation. The clinical services include the Brain Stimulation Service at NYPH (including the expert consultation service, off-label and future approved device-based therapies, and psychiatric care/evaluation for patients receiving brain stimulation for the treatment of nonpsychiatric disorders. The Brain Stimulation Service at NYPH provides expert consultation and treatment across a broad range of approved and experimental device-based therapies for depression and other disorders. This includes compassionate-use of TMS for off-label cases, and the FDA-approved clinical use of VNS for treatment resistant depression. The educational programs include CME Courses on TMS, VNS, ECT, and future brain stimulation related topics, rotations for residents/students, and mentoring for graduate students, fellows and junior faculty. The Division is an active training ground for education in the delivery of noninvasive brain stimulation for the study and treatment of psychiatric disorders with a constant flow of graduate students, residents, and postdocs and junior faculty developing K Award applications.
Where Are We Going?
The stages of medication development include: target identification, device development, pharmacokinetics, pharmacodynamics, safety/toxicology, and clinical trials. To place the new field of Emerging Brain Stimulation Techniques on the solid foundation of medical science, we must adapt these stages to device development. We need to understand where the magnetic and electrical fields go, how intense are they, and what impact do they have on brain function. We need to learn how to dose the stimulation to achieve specific effects on brain function. This may involve the use of functional neuroimaging and neurophysiology as tools to guide the development of the device to achieve clinically relevant effects on brain function.
Photos courtesy: www.magstim.com; www.neuronetics.com

|
Website designed
by: Web
Design Studio
|
Photos courtesy:
www.magstim.com; www.neuronetics.com
|